
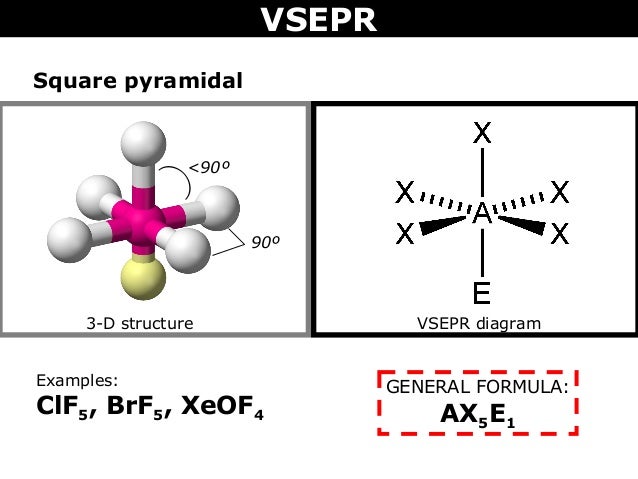
Is BrF5 follow octet rule?įor the BrF5 Lewis structure the total number of valence electrons (found on the periodic table) is 42. Hence, based on VSEPR theory, the number of 90 degree $ ] ,there is a bromide ion which has a lone pair of electrons and five bond pairs attached to the 5 Fluorine atoms. What is the total number of 90 degree angles in BrF5? What is BrF5 physical state color and shape?Ĭolorless to pale-yellow, fuming liquid with a pungent odor. If we consider PCl5 which has a trigonal bi-pyramidal structure, there is no net dipole moment as the molecule is completely symmetric around the central phosphorus atom. (BrF5), or bromine pentafluoride has a square pyramidal structure as in the first figure. According to VSEPR theory, the geometry of the molecule is octahedral but the shape is square pyramidal. There are six electron pairs around the central bromine atom, out of which five are bond pairs and one is lone pair. What is the shape of BrF5 according to Vsepr theory? Bromine pentafluoride reacts violently with water, but it will form bromic acid and hydrofluoric acid (especially when moderated by dilution with acetonitrile), simple hydrolysis products: BrF5 + 3 H2O → HBrO3 + 5 HF. How many protons are in the element bromine Br )?ģ5 What is hybridization and shape of the following molecules I BrF3 *? How do you find the hybridization of BrF3? In BrF5, there are 5 bonding pairs of electrons and one non-bonding pair of electrons. Why is BrF5 square pyramidal?īromine has 35 electrons in atomic structure he can share his 5 electron with fluorine n makes brf5 fluorine has 7 electrone in his outer orbital so he can share 1 eleceone with bromine n complete his orbital by taking 1 electron from bromine but bromine is more powerful than fluorine so he can not gain his electron … How many electron pairs are in BrF5? The molecular geometry of BrF5 is square pyramidal and its electron geometry is octahedral. It is a T-shaped molecule with a bond angle of 86.2°. What is the hybridization of bromine in BrF3?īromine Trifluoride or BrF3 is a strong fluorinating agent, and its central atom has sp3d hybridization.

What is the hybridization of nh3 molecule?.How many bond pairs and lone pairs are present in BrF5?.What is the smallest bond angle in BrF5?.What is the total number of 90 degree angles in BrF5?.What is BrF5 physical state color and shape?.What is the shape of BrF5 according to Vsepr theory?.What is hybridization and shape of the following molecules I BrF3 *?.How many protons are in the element bromine Br )?.How do you find the hybridization of BrF3?.What is the hybridization of bromine in BrF3?.


 0 kommentar(er)
0 kommentar(er)
